Fatty Acid Profile of Flaxseed
Flaxseed contains a mix of fatty acids. It is high in polyunsaturated fatty acids (73%), moderate in monounsaturated fatty acids (18%), and low in saturated fatty acids (9%). The saturated fat level of flaxseed is similar to that of canola. Flaxseed is a rich plant source of alpha-linolenic acid (ALA), an essential fatty acid in the human diet and the parent fatty acid of the omega-3 family, as shown in Figure 1. ALA is converted to two main long-chain fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in a series of enzymatic reactions (below). ALA has been shown to modulate eicosanoid synthesis,[2] and its concentration in breast milk exceeds that of DHA, suggesting a particular requirement for ALA by infants.[3, 4]
| TABLE 1 Nomenclature of Major Families of Fatty Acids[a] |
|||
|---|---|---|---|
| Parent Compound | Number of Double Bonds | Family Name[b] | Structural Abbreviations[c] |
| Oleic acid | one | Omega-9 (ω-9) | 18:1n-9 or 18:1ω-9 |
| Palmitoleic acid | one | Omega-7 (ω-7) | 16:1n-7 or 16:1ω-7 |
| Linoleic acid | two | Omega-6 (ω-6) | 18:2n-6 or 18:2ω-6 |
| Alpha-linolenic acid[d] | three | Omega-3 (ω-3) | 18:3n-3 or 18:3ω-3 |
- Adapted from Vaisey-Genser M. In Flaxseed Health, Nutrition and Functionality. Winnipeg, MB. Flax Council of Canada, 1994, p.11.
- The family name denotes the position of the first double bond as the number of carbon atoms from the methyl end of the fatty acid chain.
- Number of carbon atoms: number of double bonds (fatty acid family).
- Also designated α-linoleic acid.Alpha-linolenic acid is distinct from gamma-linoleic acid [γ-linolenic acid (18: 3n-6)], which is an intermediate in the omega-6 metabolic pathway and is a major component of evening primrose, borage, and black currant oils.
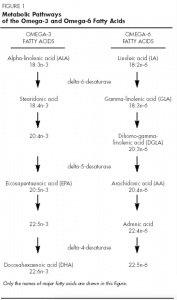
Metabolic Pathways of the Omega-3 and Omega-6 Fatty Acids
Omega-6/Omega-3 Fatty Acid Ratio of Flaxseed
ALA comprises about 57% of the total fatty acids in flaxseed, whereas the omega-6 fatty acids comprise about 16%, giving an omega-6/omega-3 ratio of 0.3: 1.1 Because the typical Western diet is high in linoleic acid and low in omega-3 fatty acids, some experts recommend replacing omega-6 fatty acids with those from the omega-3 family. Consuming flaxseed or foods rich in ALA, such as omega-3 enriched eggs derived from hens fed flaxseed, increases omega-3 fatty acid intake. This improves the dietary omega-6/omega-3 ratio.[5]
Major Families of Unsaturated Fatty Acids
There are four major families of unsaturated fatty acids (see Table 1). Oleic acid is the most prevalent fatty acid in nature and can be synthesized in the body from dietary stearic acid (18: 0).[6] Palmitoleic acid can be synthesized from dietary palmitic acid (16: 0). Oleic and palmitoleic acids are not essential in human nutrition because they can be formed from dietary precursors. Two fatty acids are required in the diets of humans because our bodies cannot manufacture them from dietary precursors: Alpha-linolenic acid, the parent compound of the omega-3 fatty acid family, and linoleic acid, the parent compound of the omega-6 family. Arachidonic acid, a metabolite of linoleic acid, is considered an essential fatty acid (EFA) only when a linoleic acid deficiency exists.[7]
Desaturation and elongation. Alpha-linolenic acid and linoleic acid are converted to their respective metabolites by a series of alternating desaturations and elongations. The desaturations add a double bond by removing hydrogen, while the elongations add two carbon atoms.
The first step in the metabolism of both EFA families is desaturation, catalysed by delta-6-desaturase. This step is followed by elongation, then desaturation (catalysed by delta-5-desaturase), then elongation and, finally, desaturation (catalysed by delta-4-desaturase). The desaturation steps tend to be slow, while the elongation steps are rapid. Thus, the tissue concentrations of gamma-linolenic acid (GLA) (18: 3n-6) and stearidonic acid (18: 4n-3) tend to be low, because they are formed slowly by desaturation and then quickly elongated to other metabolites.[8],[9]
Competition between families. Mammals cannot interconvert the omega-3 and omega-6 fatty acids. Furthermore, their metabolism requires the same desaturation enzymes, resulting in competition between the two families. An excess of one family of fatty acids can interfere with the metabolism of the other, reducing their incorporation into tissue lipids and altering their biological effects.[8], [10]
Metabolism of Alpha-Linolenic Acid (ALA)
Dietary ALA appears to have two metabolic fates. It can be desaturated and elongated to its long-chain metabolites or it can undergo ß-oxidation. Although studies of ALA ß-oxidation in humans have not been conducted, studies in animals suggest that the metabolism of ALA by ß-oxidation may contribute significantly to energy production.[11] Additional research is needed to clarify the relative importance of these metabolic pathways.
Conversion of ALA to EPA and DHA. About 15% of ALA is converted to EPA and about 5% is converted to DHA in a process that is somewhat slow in human.[5],[11] Conversion of ALA to its long-chain metabolites is affected by various dietary factors. A diet rich in linoleic acid, for example, has been shown to reduce ALA conversion by as much as 40%,10 and a high maternal intake of linoleic acid lowers EPA and DHA levels in umbilical plasma, suggesting reduced ALA conversion and availability of omega-3 fatty acids to the developing fetus.[12] Saturated and trans fatty acids also interfere with ALA desaturation and elongation,[13],[14] and in rats, ethanol inhalation results in a significant loss of liver DHA, indicating reduced ALA conversion.[15] DHA can be converted back into EPA in a reaction called retroconversion. This is believed to be a minor metabolic pathway in humans.[16]
References
- Canadian Grain Commission. Flaxseed Export Quality Data. Winnipeg, MB.
- Ferretti A and Flanagan VP. Prostaglandins Leukot Essent Fatty Acids. 1996; 54: 451-455.
- Ratnayake WMN and Chen Z-Y. Lipids. 1996; 31(Suppl): S279-S282.
- Budowski P, et al. World Review of Nutrition and Dietetics. 1994; 75: 105-108.
- Nutrition Advisory Panel Meeting: Executive Summary. Winnipeg, MB: Flax Council of Canada, 1995.
- Food Fats and Oils. Washington, DC: Institute of Shortening and Edible Oils, Inc., 1994.
- Food and Nutrition Board, National Research Council. In: Recommended Dietary Allowances, 10th ed. Washington, DC: National Academy Press, 1989, pp. 44-51.
- Horrobin DF and Manku MS. In: Omega-6 Essential Fatty Acids. Horrobin DF, ed. New York, NY: Alan R. Liss, 1990, pp. 21-53.
- Salem Jr N and Pawlosky RJ.World Review of Nutrition and Dietetics. 1994; 75: 114-119.
- Emken EA. In: Proceedings from the Scientific Conference on Omega-3 Fatty Acids in Nutrition, Vascular Biology, and Medicine. Dallas, TX: American Heart Association, 1995, pp. 9-18.
- Cunnane SC. In: Flaxseed in Human Nutrition. Cunnane SC and Thompson LU, eds. Champaign, IL: AOCS Press, 1995, pp. 99-127.
- Al MDM, et al. The Journal of the American College of Nutrition. 1996; 15: 49-55.
- Ackman RG and Cunnane SC. In: Advances in Applied Lipid Research. Padley FB, ed. London: JAI Press Ltd., 1992, pp. 167-215.
- Houwelingen AC v and Hornstra G.World Review of Nutrition and Dietetics. 1994; 75: 175-178.
- Salem Jr N, et al. Lipids. 1996; 31(Suppl): S153-S156. 16. Brossard N, et al. Am J Clin Nutr. 1996; 64: 577-586
- Brossard N, et al. The American Journal of Clinical Nutrition. 1996; 64: 577-586.
Flax Council of Canada, 465–167 Lombard Ave., Winnipeg, MB, Canada R3B 0T6
E-mail: flax@flaxcouncil.ca
Web site: www.flaxcouncil.ca
Republished with permission.
Related Flax Articles
- Nutritional Components of Flax
- The Importance of Omega-3 Fatty Acids for Adults and Infants
- Omega-3 Fatty Acids Provide Protection Against Arrhythmia









